Oxidation and Reduction
Oxidation and reduction, also known as redox, are chemical reactions in which electrons are transferred from one element to another. The substance that loses electrons is oxidized, while the substance that gains electrons is reduced. For example, when iron rusts, it is oxidized by the oxygen in the air. The iron atoms lose electrons to the oxygen atoms, forming iron oxide (rust). Oxidation-reduction reactions are also important in our bodies. For example, when we eat food, our bodies break down the food into glucose, which is then used for energy. This process is called cellular respiration, and it is a series of oxidation-reduction reactions.
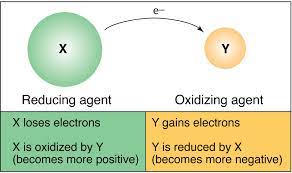
Oxidation and Reduction Definition
Oxidation: Oxidation is a chemical reaction that takes place when a substance comes into contact with oxygen or another oxidizing substance. In this process, an atom, molecule, or an ion loses one or more number of electrons in a chemical reaction. During oxidation, the state of the chemical species increases. Oxidation does not necessarily have to involve oxygen. Examples of oxidation: rust and the brown color on a cut apple.
Reduction: Reduction is the reverse of oxidation. This is a chemical reaction which involves the addition of hydrogen or any electropositive element or the removal of oxygen or any electronegative element. During reduction, the state of the chemical species decreases. Reduction does not necessarily have to involve hydrogen. Example: Copper ion undergoes reduction by gaining electrons to form copper.
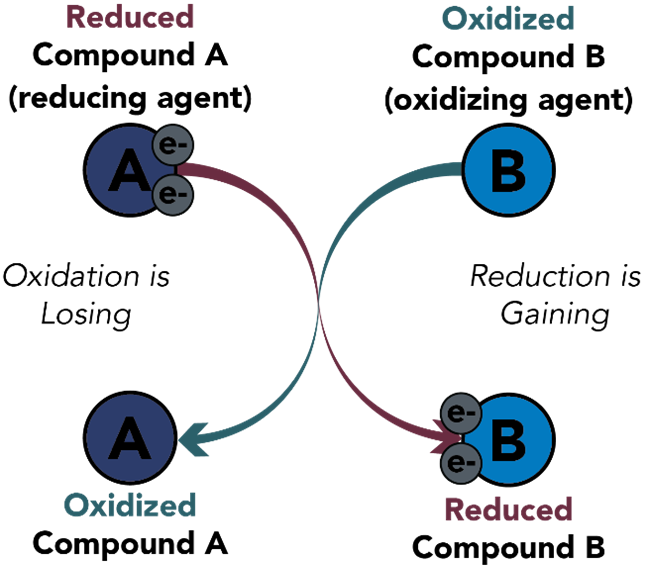
Oxidation and Reduction Reaction Examples
As mentioned above, there are many applications of the oxidation and reduction concept in the world. One of the most evident examples of this concept at play is the process of combustion. Combustion takes place when oxygen undergoes a redox reaction with any substance with flammable properties. The process of combustion has various applications ranging from digestion of food in the stomach to the thrust produced by the rocket engine. Let’s have a look at some of the Oxidation and Reduction Reaction Examples given below.
1. Reaction Between Hydrogen and Fluorine
In the hydrogen and fluorine reaction, the hydrogen is oxidized, whereas the fluorine is reduced. The reaction can be written as:
H2 + F2 → 2HF
- The oxidation half-reaction is: H2 → 2H+ + 2e–
- The reduction half-reaction is: F2 + 2e– → 2F–
The hydrogen and fluorine ions go on to combine in order to form hydrogen fluoride. Hydrogen fluoride is a poisonous and colorless gas. When Hydrogen Flouride is mixed with water, it is called Hydrofluoric acid. People who work in industries dealing with this gas should take stringent precautions in order to prevent any major damage.
2. Reaction Between Zinc and Copper
This is a metal displacement reaction in which copper metal is obtained when zinc displaces the Cu2+ion in the copper sulfate solution as mentioned in the reaction below.
Zn (s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s)
- The oxidation half-reaction can be written as: Zn → Zn2+ + 2e–
- The reduction half-reaction can be written as: Cu2+ + 2e– → Cu
Copper Sulphate is blue in colour while Zinc Sulphate is colorless. This is a very important fact that we should be aware of while conducting this metal displacement redox reaction. As soon as the blue color disappears, we can assume that the reaction has been conducted successfully. This is a common instruction given in school laboratories to make the observation easier for the students.
Oxidation Vs Reduction
In oxidation, electrons are lost, there is an increase in oxidation number and energy is released. In reduction, electrons are gained, there is a decrease in oxidation number and energy is stored. The difference between Oxidation and Reduction is briefly mentioned below.
| Oxidation Vs Reduction |
|
| Oxidation | Reduction |
| Losing electrons | Gaining electrons |
| Increase in oxidation number | Decrease in oxidation number |
| For a given compound losing hydrogen | For a given compound gaining hydrogen |
| This reaction releases energy | This reaction stores energy |
| oxidizing agents: Ozone, Bleach, peroxide | Common reducing agent is metal |
Oxidation and Reduction Equations
In the subject of chemistry, questions are asked regarding balancing the equation for a chemical reaction. Balancing an equation is the process of making sure that the number of atoms of each element is the same on either side. This has to follow the law of conservation of mass and the law of constant proportions. In the sections below let us look at the application of these concepts in the oxidation and reduction equations. The types of Oxidation and Reduction Equations are also mentioned below.
1. Balancing Oxidation-Reduction Equations
A trial-and-error approach to balance chemical equations involves playing with the equation-adjusting the ratio of the reactants and products until the below-mentioned goals have been achieved.
- The number of atoms of each element on both sides of the equation is the same and therefore the mass is conserved.
- The sum of the positive and negative charges is the same on both sides of the equation and therefore the charge is conserved. (Charge is conserved because electrons are neither created nor destroyed in a chemical reaction.)
Following are the balanced equations that can be written for the reaction between the permanganate ion and hydrogen peroxide.
| 2 MnO4–(aq) + H2O2(aq) + 6 H+(aq) |
| 2 MnO4–(aq) + 3 H2O2(aq) + 6 H+(aq) |
| 2 MnO4–(aq) + 5 H2O2(aq) + 6 H+(aq) |
| 2 MnO4–(aq) + 7 H2O2(aq) + 6 H+(aq) |
2. Redox Reactions In Acidic Solutions
Some argue that there is no need to use half-reactions to balance equations because they can be balanced by the trial and error method. The half-reaction technique becomes indispensable, however, in balancing reactions such as the oxidation of sulfur dioxide by the dichromate ion in an acidic solution.
| H+ | ||||||
| SO2(aq) | + | Cr2O72-(aq) | SO42-(aq) | + | Cr3+(aq) |
3. Redox Reactions in Basic Solutions
Half-reactions are also valuable for balancing equations in basic solutions. While using this method, you need to recognize that basic solutions contain H2O molecules and OH– ions. We can therefore add water molecules or hydroxide ions to either side of the equation, as needed.
The following equation describes the reaction between the permanganate ion and hydrogen peroxide in an acidic solution.
2 MnO4–(aq) + 5 H2O2(aq) + 6 H+(aq) ![]() 2 Mn2+(aq) + 5 O2(g) + 8 H2O(l)
2 Mn2+(aq) + 5 O2(g) + 8 H2O(l)
4. Molecular Redox Reactions
Lewis structures play an important role in understanding oxidation-reduction reactions with complex molecules. Consider the following reaction, for example, which is used in the Breathalyzer to determine the amount of ethyl alcohol or ethanol on the breath of individuals who are suspected of driving while under the influence.
3 CH3CH2OH(g) + 2 Cr2O72-(aq) + 16 H+(aq) ![]() 3 CH3CO2H(aq) + 4 Cr3+(aq) + 11 H2O(l)
3 CH3CO2H(aq) + 4 Cr3+(aq) + 11 H2O(l)




 Upcoming Government Exams, Complete Govt...
Upcoming Government Exams, Complete Govt...
 SSC Exam Calendar 2025–26 Out, Check All...
SSC Exam Calendar 2025–26 Out, Check All...
 RRB JE 2024 Notification, CBT 2 Exam Dat...
RRB JE 2024 Notification, CBT 2 Exam Dat...


